Weight per gallon 15C 8338 pounds 3782 kilograms. Water is the most common coolant.

What Are The Bubbles In Boiling Water

Solid Absorbent An Overview Sciencedirect Topics

The Best Way To Prevent Containerised Goods From Getting Moisture Damage Macandrews Door To Door Intra European Multimodal Transport Solutions
Biosphere controls on minimum temperatures During the late 1860s British experimental physicist John Tyndall based on his studies of the infrared radiation absorption by atmospheric gases concluded that nighttime minimum temperature s were dependent on the concentration of trace.
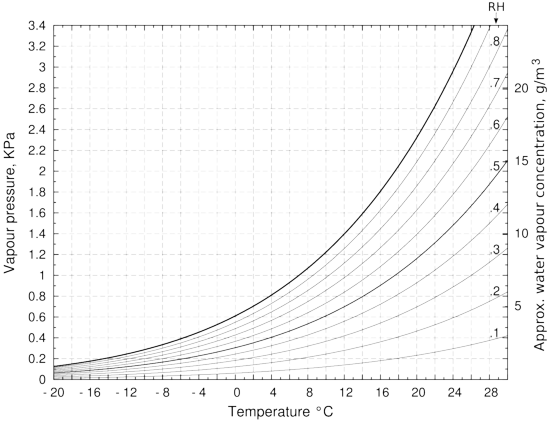
Substance that absorb water vapour. PH is a measure of how acidic or alkaline something is. Remove contact lenses if present and easy to do. In the condenser the winding tube containing the vapour is passed through either circulating air or a bath of water which removes some of the heat energy of the compressed gas.
The lowest possible energy state of a substance is defined as the absolute zero 27315 C or climate. Identification of the substancemixture and of the companyundertaking 11. For personal protection see section 8.
Any of various forms. Rinse skin with water shower. Do not allow product to reach sewage system or open waterIt is the responsibility of the waste generator to properly characterize all waste materials according to applicable regulatory entities US 40CFR26211.
Most of the naturally occurring organics have a slightly negative charge. Its high heat capacity and low cost makes it a suitable heat-transfer medium. A clear colorless odorless and tasteless liquid H2O essential for most plant and animal life and the most widely used of all solvents.
For personal protection see section 8. These dressings allow water vapour to enter keeping the area moist promoting faster healing but prevent bacteria from entering the affected area. Absorb spillage with non-combustible absorbent material.
Boiling point 100C 212F. Boiler water chemicals. Product identifier Acetone EC No.
The pH scale ranges from 114 with a pH of 7 being neutral the pH of pure water. The cooled vapour is passed through an expansion valve to an area of much lower pressure. Do not discharge into drains water courses or onto the ground.
Absorb with a noncombustible absorbent material such as sand or earth and containerize for disposal. The coating which is made from vertically aligned carbon nanotubes CNT grown on chlorine-etched aluminium foil can absorb 99995 percent of visible light. Freezing point 0C 32F.
If the pH is higher than 7 the substance is alkaline as is the case with soaps bleach and ammonia. Foam dressings absorb exudates from the wounds surface creating an environment that promotes faster healing. Details of the supplier of the safety data sheet Thornton Ross Ltd Linthwaite Huddersfield HD7 5QH.
Clean contaminated area with oil-removing material. Methods and material for containment and cleaning up Methods for cleaning up. Specific gravity 4C 10000.
Absorb spillage to prevent material damage. Do NOT induce vomiting. Absorb spillage with oil-absorbing material.
Wash contaminated clothing before reuse. The concentration of the substance and the kind of substance that is added depend upon the necessary decrease or increase of the pH. Reference to other sections References.
Methods and material for containment and cleaning up Methods for cleaning up. Temperature-dependency of the heats of vaporization for water methanol benzene and acetone The enthalpy of vaporization symbol H vap also known as the latent heat of vaporization or heat of evaporation is the amount of energy enthalpy that must be added to a liquid substance to transform a quantity of that substance into a gas. Do not discharge into drains water courses or onto the ground.
It is usually used with additives like corrosion inhibitors and antifreezeAntifreeze a solution of a suitable organic chemical most often ethylene glycol diethylene glycol or propylene glycol in water is used when the water-based coolant has to withstand temperatures below 0. Rinse cautiously with water for several minutes. If pH is lower than 7 a substance is considered acidic think vinegar or lemon juice.
Relevant identified uses of the substance or mixture and uses advised against Household Solvent 13. As the vapour expands it draws the energy of its expansion from its surroundings or the medium in contact with it. For waste disposal see section 13.
What Is Soil pH. Due to that they can absorb oxygen molecules because these carry a slightly positive charge. Reference to other sections References.
Water wôtər wŏtər n. Methanol RCRA waste code U154.
/thai-puff-ball-mushroom-soup---barometer-earthstars--hygroscopic-earthstar--false-earthstar--astraeus-hygrometricus---mushroom-657663470-5af1ab958e1b6e0039011b8c.jpg)
Hygroscopic Definition In Chemistry
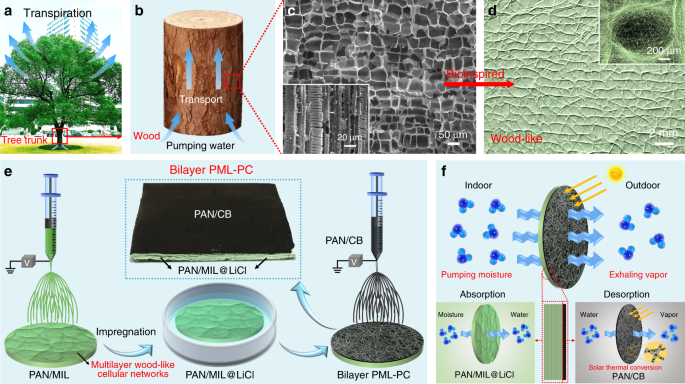
Super Hygroscopic Nanofibrous Membrane Based Moisture Pump For Solar Driven Indoor Dehumidification Nature Communications

Difference Between Desiccant And Deliquescent Compare The Difference Between Similar Terms

Hygroscopic Material An Overview Sciencedirect Topics
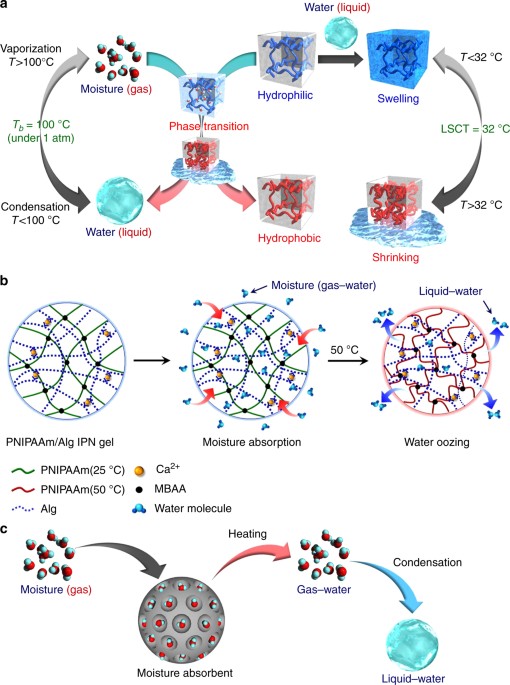
Thermo Responsive Gels That Absorb Moisture And Ooze Water Nature Communications

Conservation Physics The Interaction Of Water Vapour With Paper In Small Spaces

2a Solar Energy And The Water Cycle
1
