Equation 4 where A is the measured absorbance of the solution. A standard curve of optical density vs.
Chem 125 Experiment Ii
Lab 9 Determination Of Allura Red Concentration In Mouthwash

Graph Of Correlation Between Concentration And Absorbance Of Quercetin Download Scientific Diagram
Keep this quantity in mind.
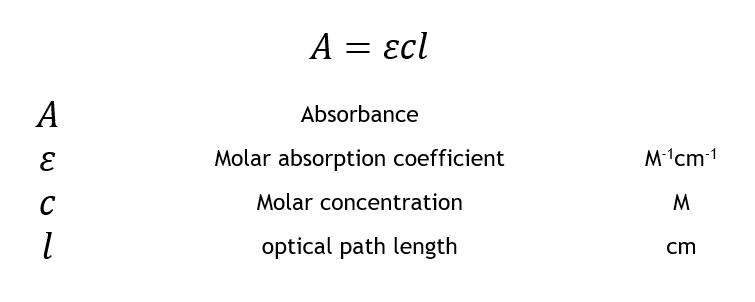
Relationship between the concentration of a solution and the absorbance. Solute concentration then the trend or relationship can be modeled by calculating the best-fit line or curve by regression analysis see A Painless Guide to Statistics Do not connect the dots when the measurements. According to Beer-Lambert law the absorbance of light as it passes through a solution is directly proportional to the path length of light. The Beer-Lambert law or Beers law is the linear relationship between absorbance and concentration of an absorbing species.
Beers Law AEbc helped to develop the linear equation since absorbance was equal to y Eb was equal to m and the concentration c was equal to the slope x in the equation ymxb. Here a is the proportionality constant molar absorptivity if concentration units are molarity. You will prepare a stock solution from which four solutions of known concentration standard solutions will be made.
And c is the concentration of a given solution. To determine the standard curve of absorbance versus concentration several steps were necessary. In addition knowledge of.
First serial dilutions of the stock solution were made. In other words the more material in the solution absorbs the light the less light will get through. A second vial containing 45 ml and 05 ml of stock solution and water respectively was made.
If all the light is absorbed then percent transmittance is zero and absorption is. But what was the concentration of your ORIGINAL solution. Unknown m gml Measured A260 50 m gml 10 A260 Since there is a linear relationship between absorbance and DNA concentration we can use some simple algebra and reformulate as follows.
Second find the relationship between absorbance and concentration. Concentration will show a linear relationship. Practically speaking its what youre the most interested in.
If cells of different path lengths are available testing if this relationship holds true is one way to judge if absorption flattening is occurring. Consequently the absorbance can also be given in terms of the percentage transmittance. Molar and percent extinction coefficient ε.
The result is that P2-. Absorbance is defined as. Absorbance A ε x c x l c concentration of the sample in Molesliter l length of the light path through the solution in cm and ε molar extinction coefficient To determine the absolute concentration of a pure substance a standard curve is constructed from the known concentrations and using that standard curve the absorbance reading of the unknown concentration was determined.
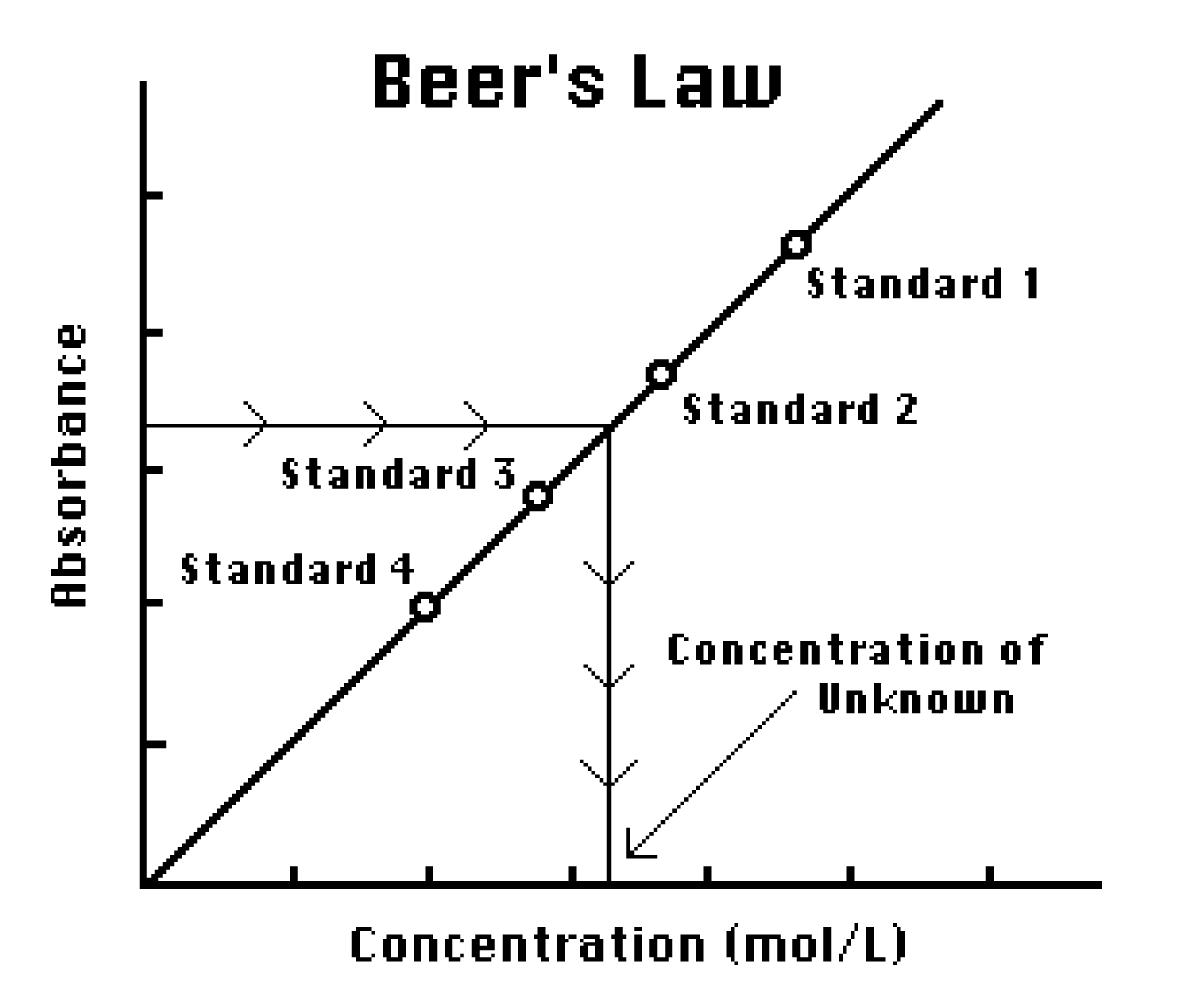
In the BeerLambert law varying concentration and path length has an equivalent effectdiluting a solution by a factor of 10 has the same effect as shortening the path length by a factor of 10. The operator can measure the response of the unknown and using the calibration curve can interpolate to find the concentration of analyte. This is the E value EAC if L1 so the slope of the standard curve gives you E.
The Beer-Lambert law states that there is a linear relationship between the concentration and the absorbance of the solution which enables the concentration of a solution to be calculated by measuring its. This relationship is expressed by Beers Law Equation 1. This law states that the absorbance of a light absorbing material is proportional to its concentration in solution.
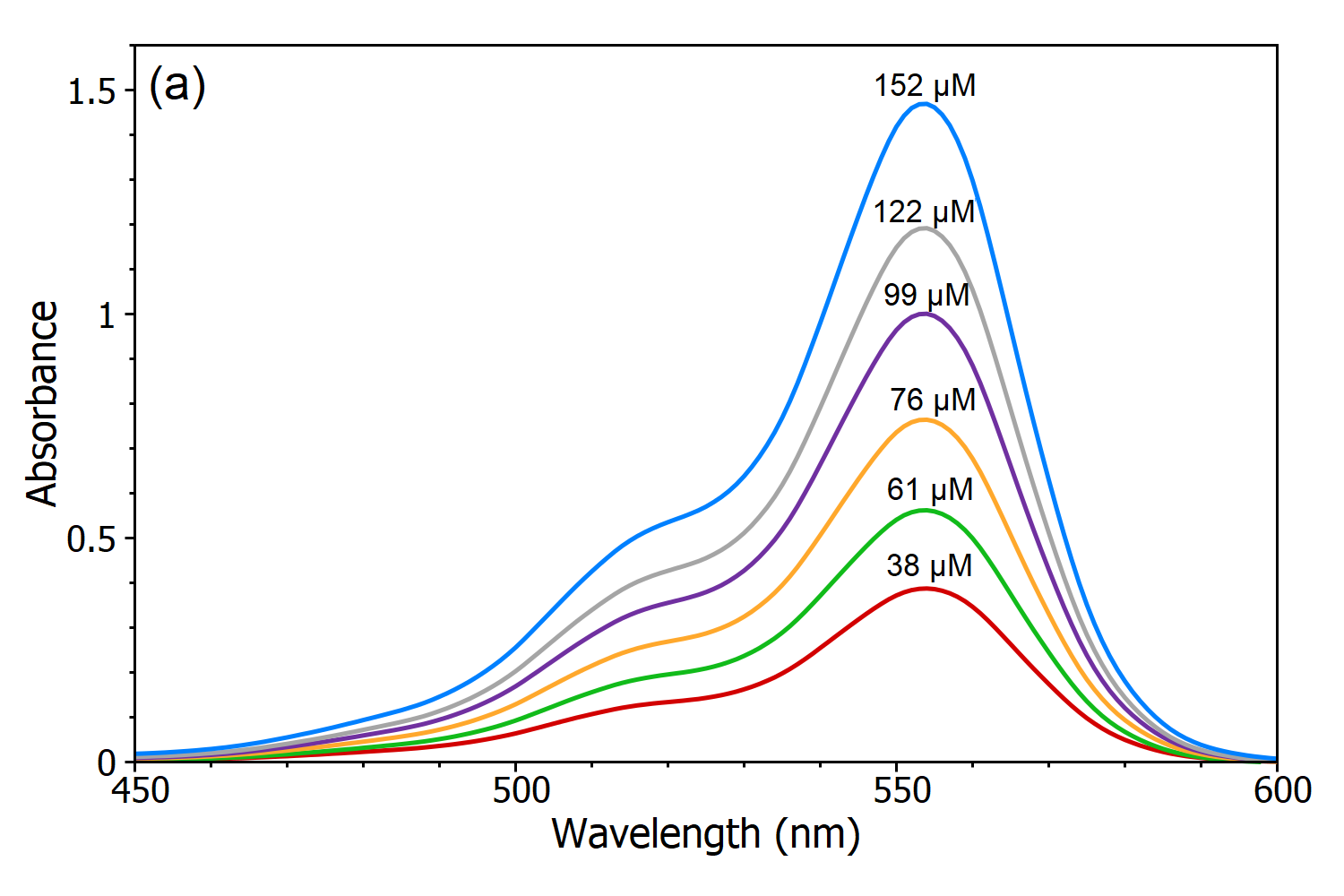
The relationship between absorbance and concentration was directly proportional with a molar absorptivity of 281 M-1cm. Beers law says that the relationship between the absorbance of the chromophore and its concentration is linear allowing construction of a standard curve by plotting absorbance versus concentration such as shown in Figure 1. A a b c where A is the measured absorbance a is a wavelength-dependent absorptivity coefficient b is the path length and c is the analyte concentration.
If all the light passes through a solution without any absorption then absorbance is zero and percent transmittance is 100. When a graph of absorbance vs. The linear relationship between absorbance and concentration displays that absorbance depends on the concentration.
The relationship between molar extinction coefficient ε. Mean price of computer memory over time. Not all substances obey the linear Beer-Lambert law over all concentration ranges.
Mathematically Beers law is expressed as shown in equation 4. A species in solution and is the relationship used when making quantitative measurements. 10 concentration in mgml.
A ε l c Equation 1 A is the absorbance observed. This is the line of best fit through your data. Lastly C is the molar concentration of the chromophore molL used for the measurement.
The relationship between absorbance and concentration c is proportional. Usually the more concentrated a substance the more light will be absorbed. The concentration is simply the moles L-1 M of the sample dissolved in the solution and the length is the length of the cuvette used for the absorbance measurement and is typically 1 cm.
Therefore you will construct a calibration curve that will provide the relationship between concentration and absorbance under the conditions used for the analysis. Concentration of the absorbing compound in solution. Molar 10 ε.
Once you have measured E you can find the concentration of any solution of that compound by measuring Absorbance at the same. Thus one can use the following formula to determine the DNA concentration of a solution. Relationship is known as Beers law and is expressed mathematically as A abc.
For most analyses a plot of instrument response vs. Concentration is plotted for the standard solutions a direct relationship should result as. And then its easy.
Using the pipettes from step 1 5 ml of stock solution was placed in one vial without any water. Is the molar absorptivity of the substance l is the path width for the cell and c is the concentration. When Absorbance is plotted against concentration the slope of the line is the relationship between concentration and Absorbance.
There is a relationship between concentration and absorbance. The general Beer-Lambert law is usually written as. Remember you dilluted it once so you can use the Dilution Equation.
This relationship is expressed by the Lambert-Beer law which is more commonly known as Beers law. The absorbance of each will be measured. ε is the extinction coefficient also referred to as the.
Now this is the absorbance of your DILUTED solution. Absorbance How does the absorbance tell you the concentration. In more general use a calibration curve is a curve or.
Concentration dependence of the absorbance at the oscillator position 1700 cm 1 black curve compared to the normalized absorbance green line integrated from 1003000 cm 1 to the normalized integrated absorbance calculated between 16001800 cm 1 red line and to the normalized integrated absorbance calculated between 16001840 cm 1 blue line. B is the path length of radiation going through the solution and c is the concentration of the solution. The data obtained reinforced the connection between the color of a solution and the wavelengths of light which the solution absorbs and reflects.
The relationship between absorbance and transmittance is illustrated in the following diagram. Percent is as follows. Percent molecular weight of protein Still other sources provide protein absorbance values for 01 mgmL solutions as this unit of measure is more.
If however the series represents independent measurements of a variable to show a trend eg. You have an absorbance and you have a straight line equation that relates absorbance to concentration.

Investigation 1 What Is The Relationship Between The Concentration Of A Solution And The Amount Of Transmitted Light Through The Solution Ppt Video Online Download

Solved Se The Best Answer 1 In General Terms What Is The Chegg Com
Beer S Law Theoretical Principles

Beer Lambert Law Transmittance Absorbance Edinburgh Instruments
Beer Lambert Law Transmittance Absorbance Edinburgh Instruments
Untitled Document
Determining The Concentration Of A Solution Beer S Law Vernier

Absorbance Measurements The Quick Way To Determine Sample Concentration Eppendorf Handling Solutions
