Curve is a plot of analytical signal eg. From the graph note the wavelength of maximum absorbance for this solution.

Standard Calibration Curve Of Absorbance Against Concentration Of Download Scientific Diagram
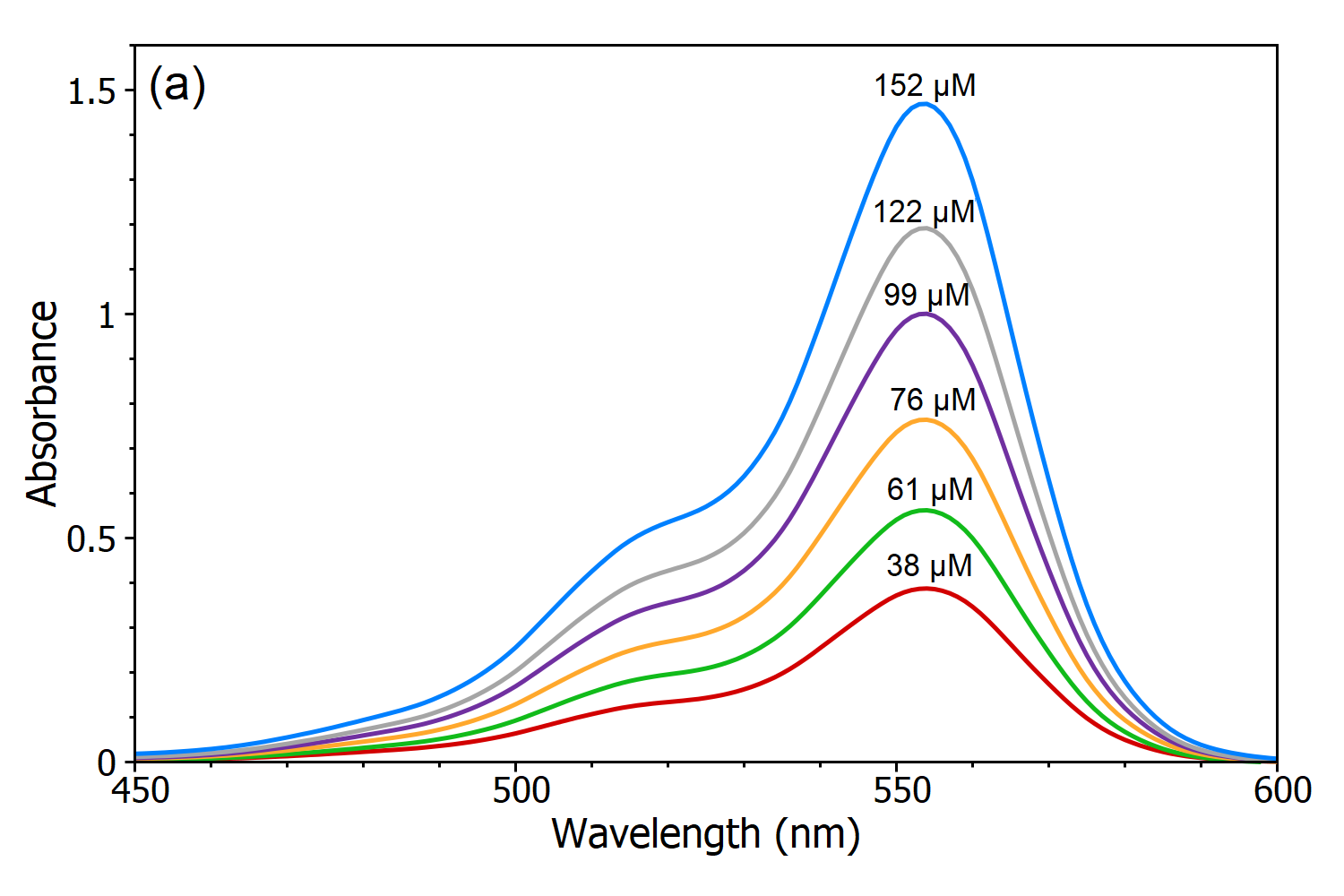
Beer Lambert Law Transmittance Absorbance Edinburgh Instruments

Solved The Following Absorbance Vs Concentration Of Nitrite Graph Is Obtained Solutioninn
This will enable you to plot a graph of Velocity of reaction absorbance units per sec against Substrate concentration M.

Plot a graph of absorbance against concentration. Once the Beer-Lambert law is obeyed a plot of absorbance against concentration will give a straight line the slope of which is the molar absorptivity ε length. 3 Measure absorbance at suitable time intervals for 5 minutes or until there is little change in reaction. A graph of absorbance vs concentration is called a Beers Law curve in honor of the chemist who first discovered the relationship between absorbance and concentration.
From January 2021 many browsers will no longer. Draw a tangent to each line of best fit at time t 0 s. Plot the results as absorbance against wavelength.
5 Plot a graph of absorbance against time. Record your results in a suitable table. Risk Assessment Hazard Risk Safety Precaution In emergency Risk Level Scalpel Cuts from sharp object Cut away from fingers.
In log-log calibration the logarithm of the measured signal A y-axis is plotted against the logarithm of concentration C x-axis and the. Prepare solution of benzoic acid in ether containing 20 40 60 80100 and 120 mgl. This value was measured on the graph using a ruler to be approximately 100 x 10-6 M.
Table 2 Figure 2. Plot absorbance or mV readings for the 100- 10- and 1-mgL standards on semi-logarithmic graph paper with concentration on the logarithmic x axis. It is common to plot a calibration curve for a colorimeter by making up solutions of the coloured substance of known concentration and then measuring the absorbance of each under the same conditions as you will do the experiment.
Using the formula it was determined to be 976 x 10-6 M. Use the graph to determine the initial rate of reaction. Note that each is a separate dilution of a 10mgml solution.
Solutions are being studied to allow Flash games being playable again on browser. Alternatively for samples which scatter light absorbance may be defined as the negative logarithm of one minus absorptance as measured on a uniform sample. Early days people are using transmittance but these days people practicing absorbance its is just convention.
This is the calibration curve. Analysis of results 1. Use the graph to determine the initial rate of reaction for each.
Figure 4 is Beers Law curve for the absorbance of an iron-salicylate complex the substance prepared in todays experiment plotted against different concentrations. On a page of graph paper plot the Absorbance readings of the BSA samples on the Y- axis as a function of BSA ie. Other minimum at approximately 2765 nm aspoint D and highest maximum at approximately 272 nm as point C.
You then plot a graph of absorbance against concentration to give your calibration curve. Wavelength λ Absorbance 235 0399 240 0592 245 0574 250 0442 255 0307 260 0228 265 0148 270 0115 Result. If a test sample produces an absorbance of 14 the corresponding two concentrations calculated by linear curve r 092255796 and nonlinear curve r 099993479 have a huge difference 199955 VS 114898.
The term is used in many technical areas to quantify the results of an experimental. As absorbance directly give idea of concentration and reciprocal so people start using it. Calculate the gradient of each tangent in order to deduce the initial rate of each reaction at each concentration.
Plot absorbance againstwavelength and record wavelength of minimum at approximately 2675 nm as point B. 4 Discard the content of the cuvette and rinse with distilled water. How to calculate concentration from absorbance in excel Oh Nooooo.
The unknown concentration of sample. A Job plot otherwise known as the method of continuous variation or Jobs method is a method used in analytical chemistry to determine the stoichiometry of a binding event. The unknown concentration B in these terms was found to be 311 x 10-6 gL.
6 Measure the absorbance of the solution of unknown concentration using the colorimeter. Graphs for a zero-order reaction a rate plotted against time and b concentration plotted against time. From the graph find the maximum velocity and half it ie.
Rinse the cuvette with distilled water and repeat for each concentration. The variables of the Michaelis-Menten equation can be manipulated to produce an equation as a function of the inverse of the substrate concentration as related to the inverse of the rate of the reaction. From January 2021 many browsers will no longer support Flash technology and some games such as Super Smash Flash 2 may not work.
It is represent a graph where concentration is plotted against absorbance then a straight line Beers Law is fit to the data that you obtained and the resulting equation is used to convert absorbance of the unknown sample into concentration. It should be possible to plot each concentration as a different line on the same axes. Concentration is curvilinear and it plateaus as it approaches V max.
Doing this produces a linear graph known as the Lineweaver-Burk plot. For each substrate concentration calculate the rate velocity of reaction Absorbance units produced per unit Time. 3 5 Draw the line of best fit through the data points.
Therefore the concentration used in the plot is for the dilution. If the plot results in a straight line then the reaction is first order and the rate constant k is equal to the slope of the line ie. X unknown Concentration mM Y Absorbance at 420 nm 0.
Which states that the absorbance of a solution is directly proportional to its concentration c as long as the solution path length l and the wavelength of measurement are constant. 7 Use the calibration curve to determine the concentration of this solution. Plot a graph of absorbance against time.
Draw a line of best fit. 4 Plot the absorbance vs concentration for each standard solution on a graph. In addition concentration in gL were calculated and included in Table 2.
Measure the absorbance for each solution. The method is named after Paul Job and is also used in instrumental analysis and advanced chemical equilibrium texts and research articles. Absorbance is defined as the logarithm of the ratio of incident to transmitted radiant power through a sample excluding the effects on cell walls.
This is the initial gradient of the graph and should be the steepest part. Another commonly-used plot in examining enzyme kinetics is the Lineweaver-Burk plot in with the inverse of the reaction rate 1r is plotted against the inverse of. A higher absorbance indicates higher pigment concentration and hence a more permeable membrane.
I believe that the equation should give me direct calculation of unknown concentration by putting absorbance y in the equation ie. Determine absorbance of these solutions in a. This is an example to show you how different standard curves affect result.
Order with respect to A is plot a graph of lnA versus time. Protein concentration in each reaction mixture on the X-axis. Plot a graph of volume of hydrogen produced on the y-axis against time in seconds for each hydrochloric acid concentration.
Job first published his method in 1928 while studying the associations of ions. Then make an XY scatter graph putting concentration on the X horizontal axis and signal on the Y.

1 2 Beer S Law Chemistry Libretexts

Beer Lambert Law Transmittance Absorbance Edinburgh Instruments

Standard Curve Of Absorbance Versus Concentration Of Oil In Octanol Download Scientific Diagram

Experimental Determination Of Reaction Rates Introduction To Chemistry

Calibration Graph Of Absorbance Vs Concentration Download Scientific Diagram
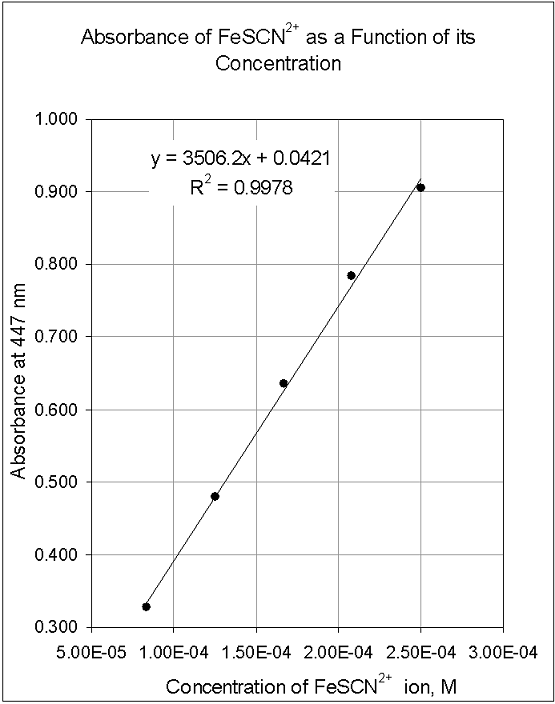
Plotting A Calibration Curve
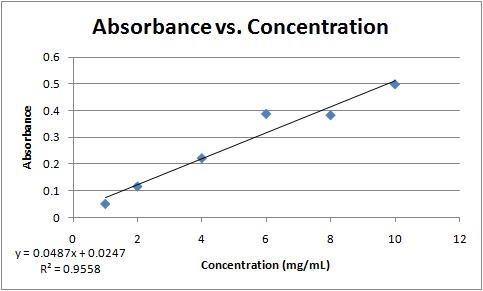
Question 7f9f2 Socratic

The Absorbance Versus Concentration Graph Of Chlorpyrifos Download Scientific Diagram
