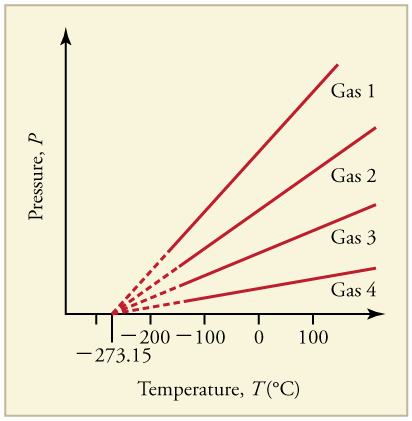
Any real gas actually condenses to a liquid or a solid at some temperature higher than absolute zero. This is the lowest possible temperature in the universe known as absolute zero.

Lab 12 The Ideal Gas Law
Ideal Gas Law Boundless Physics

Absolute Zero Definition Facts Britannica
Boyles law Charless law and Avogadros law.
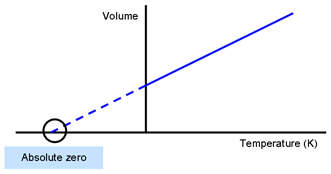
Absolute zero in the ideal gas laws. Absolute zero is the lowest limit of the thermodynamic temperature scale a state at which the enthalpy and entropy of a cooled ideal gas reach their minimum value taken as zero kelvinsThe fundamental particles of nature have minimum vibrational motion retaining only quantum mechanical zero-point energy-induced particle motionThe theoretical temperature is determined by extrapolating the. It is convenient to define an arbitrary reference state. Combining these four laws yields the ideal gas law a relation between the pressure volume temperature and number of moles of a gas.
Such a relationship is referred to as the equation of state. The coldest theoretical temperature is called absolute zeroIt is the temperature where the thermal motion of particles is at its minimum not the same as motionless. P is the pressure exerted by an ideal gas V is the volume occupied by an ideal gas T is the absolute temperature of an ideal gas R is universal gas constant or ideal gas constant n is the number of moles amount of gas.
An ideal gas is taken usually as reference for the thermodynamic properties of a real fluid. 3793 SCF at standard conditions of 60F and 147psia see Reference 1. The gas laws of Boyle and Charles and Gay-Lussac can be combined into a single equation of state.
Ideal Gas Law Units. When we use the gas constant R 831 JKmol then we have to plug in the pressure P in the units of pascals Pa volume in the units of m 3 and the temperature T in the units of kelvin K. If a gas contracts by 1273 of its volume for each degree of cooling it should contract to zero volume at a temperature of 273C.
When we use the gas constant R 0082 LatmKmol then pressure should be in the units of atmospheres atm volume in the units of litres L and the temperature T in the units of kelvin K. Temperature is the property of matter which reflects the quantity of energy of motion of the component particles. An ideal gas is a hypothetical construct that may be used along with kinetic molecular theory to effectively explain the gas laws as will be described in a later module of this chapter.
The Ideal Gas Law is a simple equation demonstrating the relationship between temperature pressure and volume for gases. Charles Law Boyles Law and Avogadros Law all of which will later combine into the General Gas Equation and Ideal Gas Law. Or a real gas that behaves like an ideal gas.
As such however it is extremely useful. Derivation of Ideal Gas Law. It is a comparative measure of how hot or cold a material is.
The gas laws consist of three primary laws. P is the pressure V is the volume N is the number of moles of gas R is the universal gas constant and T is the absolute temperature. This extrapolation of Charles Law was the first evidence of the significance of this temperature.
Determine the average molar mass of air. Ideal Gas Practice Problems. The gas laws are a set of intuitively obvious statements to most everyone in the Western world today.
Gay-Lussacs Law is an ideal gas law where at constant volume the pressure of an ideal gas is directly proportional to its absolute temperature. The concept of absolute zero as a limiting temperature has. Under normal conditions temperature 0 C and atmospheric absolute pressure 100 kPa the air density is 128 kgm³.
In the metric system of units we must specify. As a result the absolute entropy of any element or compound can be measured by comparing it with a perfect crystal at absolute zero. The universal gas constant R is a number that satisfies the proportionalities of the pressure-volume-temperature relationship.
Although all the calculations presented in this module assume ideal behavior this assumption is only reasonable for gases under conditions of relatively low pressure and high temperature. Therefore the ideal gas law is only an approximation to real gas behaviour. And yet someone had to notice these relationships and write them down.
Therefore a relation between these quantities and the mass of a gas gives valuable information about the physical nature of the system. In an ideal gas there is no molecule-molecule interaction and only elastic collisions are allowed. The relationship between the pressure of an ideal gas and its Kelvin temperature is expressed in Gay-Lussacs law.
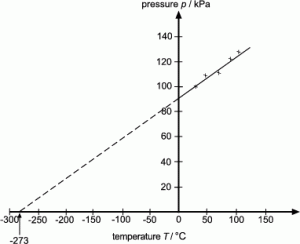
Check this value is close to the accepted value of 273C. Charless Law identifies the direct proportionality between volume and temperature at constant pressure Boyles Law identifies the inverse proportionality of pressure and. The ideal gas law can easily be derived from three basic gas laws.
Lab 10 - The Ideal Gas Law Introduction The volume of a gas depends on the pressure as well as the temperature of the gas. Because the enthalpy of an ideal gas does not depend on pressure its variation between two states depends only on temperature. These specific relationships stem from Charless Law Boyles Law and Gay-Lussacs Law.
Its hard to believe that there was ever a time when they werent understood. At absolute zero h 0 0 mT 0 h 1 mT 1. It is a good approximation to the behavior of many gases under many conditions although it has several limitations.
Extrapolation to Zero Volume. 4 The enthalpy of an ideal gas H 0 can be set at zero for T T 0. Picking any co-ordinate of h and t from the line of best fit and substituting into the equation will give a value of absolute zero.
Student exploration ideal gas law answers Student exploration ideal gas law answers. LatexPV nRTlatex where P is the pressure of a gas V is its volume n is the number of moles of the gas T is its temperature on the kelvin scale and R is a constant called the ideal gas constant or the universal gas constant. Typically for air quality regulatory calculations for natural gas and its components an ideal gas is assumed and compressibility factors Z-factors are not used.
From the given air density we know that the mass of one cubic meter of air is 128 kg. An extrapolation to zero pressure is necessary because real gases condense to liquids and solidify before reaching absolute zero. Gas Properties - PhET Interactive Simulations.
The entropy data are therefore given as absolute numbers S o not entropies of formation S o f. Also be aware that the temperature given in the equation of state must be an absolute temperature that begins at absolute zero. The ideal gas law conversion factor used above is based on the relationship of 1 lb-mole of an ideal gas occupies approx.
The ideal gas law is the equation of state of a hypothetical ideal gas an illustration is offered in. Combined these form the Ideal Gas Law equation. Click the Reset button and enter the problem data into the calculator.
In other words Gay-Lussacs Law states that the pressure of a fixed amount of gas at fixed volume is directly proportional to its temperature in kelvins. The third law defines absolute zero on the entropy scale. Introduction The three fundamental gas laws discover the relationship of pressure temperature volume and amount of gas.
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanicsA thermodynamic temperature reading of zero is of particular importance for the third law of thermodynamicsBy courtesy it reported on the Kelvin scale of temperature in which the unit of measure is the kelvin unit symbol. Student exploration ideal gas law answers.

Charles Law Absolute Zero Chemistrygod

Absolute Zero And The Ideal Gas Law Absolute Zero And The Ideal Gas Law Objective To Discover The Estimation Of Absolute Zero Which Is Resolved In Course Hero
Topic Ideal Gas Laws Archives Tutormyself Chemistry

Ideal Gas Law Ideal Gas Equation Derivation Solved Examples

Episode 602 Ideal Gases And Absolute Zero Iopspark

Absolute Zero Temperature Definition Video Lesson Transcript Study Com

Ideal Gas Equation And Absolute Temperature Boyle S Law Derivation

Phase Changes Physics